-
Commercialization Education -
FFMI fastPACE -
Frankel Cardiovascular Center Innovation Program -
Rogel Cancer Center Innovation Program -
Global Health Commercialization Competition -
Biomedical Innovation 101 -
Certificate in Biomedical Innovation & Entrepreneurship -
Fast Forward Medical Innovation Fellowship Program -
Biotech Career Development Program -
Drug Discovery & Development Course -
Innovation Studio -
Oncology Drug Discovery & Development (3D) Workshop -
FFMI fastPACE Train-the-Trainer -
Intellectual Property in the Academic Setting -
Drug Discovery and Development Course -
Medical Device Regulation -
Idea to Impact Webinar Series -
6 Tips for Customer Discovery -
Interacting with Industry -
Ask the Experts: A Biomedical Innovation Forum
-
-
Business Development -
Michigan Biomedical Venture Fund

Fast Forward Medical Innovation
We work to enable and catalyze productive academic-industry partnerships, leveraging U-M faculty innovations to improve healthcare.
The Fast Forward Medical Innovation Business Development (BD) team facilitates all forms of Academic-Industry collaborations. We utilize expert understanding of research contracting, budget, and negotiating to strengthen industry relationships and ensure successful project execution.

FFMI Business Development (BD), under the guidance of Ingrid Qemo, BD Industry Alliance Manager, played a pivotal role in facilitating a significant partnership recently announced between Atonoco, a biotechnology firm based in France, and the University of Michigan.
Together, they are embarking on a Phase 1-2a clinical trial aimed at evaluating a promising therapy for bladder cancer. Heading this project are Benjamin Viglianti, M.D., Ph.D., Clinical Associate Professor of Radiology, and Peter Scott, Ph.D., Professor of Radiology.
The FFMI BD team provided crucial support to both Atonoco and the University of Michigan in planning and executing a two-part contract. The first part, which involves FDA submission, has been signed, with submission to the FDA expected by the end of calendar year. This comprehensive agreement spans up to two years of preparatory work necessary for FDA approval, followed by the clinical trial itself.
Working closely with Dr. Viglianti and Dr. Scott, the FFMI BD team successfully secured vital resources from U-M's MICHR IND/IDE Lifecycle Maintenance (MIAP) program, led by Mitch Seymour and Jeanie Wright, as well as Melanie Fee and Roxane Collins from the Clinical Trials Support Unit, to ensure thorough preparation for FDA submission.
Currently, the FFMI BD team is actively preparing the budget and contract for the upcoming clinical trial agreement. The trial aims to enroll its first patient by the first quarter of 2025, representing a significant step forward in advancing this potentially groundbreaking therapy for bladder cancer.
Collaborators can use this searchable database to find U-M faculty members whose expertise aligns with their needs. Additionally, they can explore faculty-proposed project ideas and access information on available lab and clinical resources. If you are interested in working with industry and would like to be featured on this dashboard, please complete this brief survey or reach out to the BD team directly at [email protected]. We look forward to working with you!
The BD team provides 1:1 support to faculty researchers and study teams based within the Medical School who have an interest in collaborating with Industry. We help to identify partnerships and maintain clear communication with the sponsor from project conception through execution. We provide business guidance to faculty to ensure alignment of expectations, reduction of risk, and overall partnership structures that are win-win for the company and the PI.
Our library of educational videos and list of frequently asked questions provide in-depth information on how the Business Development team helps researchers work with industry and other external partners, towards the ultimate goal of impacting patient health.
Mike Ranella, MBA, MPH, Senior Director of Business Development, provides an overview of the Business Development team, including what business development is and how the team can support faculty as they partner with industry.
Debra Grega, MBA, PhD, Former Director of Business Development, talks about the difference between sponsored research and OSA, along with the basics of each.
Hear from Mike Ranella, MBA, MPH, Senior Director of Business Development, and Sheryl Flanagan, PhD, Data Protection Coordinator at the U-M Medical School, about the policies governing human data and biospeciman transfers.
Learn about how U-M and the FFMI Business Development team support industry-sponsored clinical trials from Emilija Mitrikeska, MD, Associate Director of Business Development.
Learn about the variety of ways you can partner with industry from Shelby Unsworth, PhD, Associate Director of Business Development.
Assistant Director of Regulatory Affairs, David Mulder, BA, CRA, provides an overview of consulting policy. In this video, he'll walk you through each step of a typical consulting engagement and give some important points to consider at each stage of the consulting process.
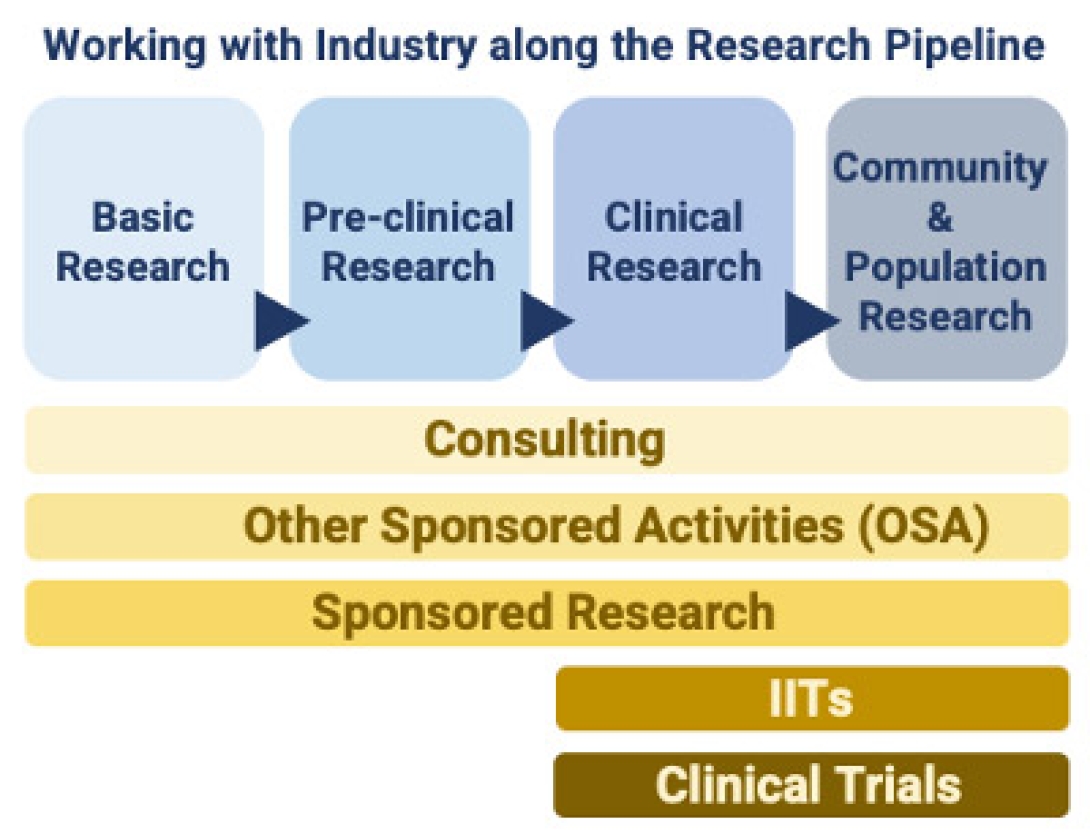
What are the different ways of working with industry on my research?
There are many ways that our faculty can partner with industry sponsors all along the translational research pipeline including consulting, other sponsored activities (OSA), sponsored research, Investigator Initiated Trials, and Industry Initiated Clinical Trials. The FFMI BD team is here to help facilitate the successful execution of each of these different types of partnerships.
Should I negotiate the budget and contract for my industry project?
We recommend a team approach when engaging and negotiating with industry. Although it is not required to work with the FFMI BD team when negotiating, we are here to support all aspects of crafting a win-win deal between your research team and the industry partners.
Can the FFMI BD team help me with ITT concepts with industry?
The FFMI BD team provides support to meet the specific needs of your project. We can provide company contacts, or help to negotiate deals for sponsorship, access to study drugs, or in-kind services such as sequencing. Reach out to the FFMI BD team to explore your options.
Why do industry partners want to work with academic researchers?
Industry partners work with academic researchers who have specific expertise, tools, or resources that complement their in-house capabilities and support their strategic goals.
Financial support for research
One of the biggest benefits of working with industry is the financial support they provide for your research. This alternate funding stream can be key as federal funding is becoming more and more competitive to obtain.
Access to investigational drugs, devices, assays, and data
Working with Industry may help you gain access to new drugs, devices, or assays to test in your lab. By working with Industry on developing products, you may also gain access to important data from these products that may support your research goals.
No, only personnel with Regental Signature Authority, such as ORSP or U-M Legal, can sign on behalf of University of Michigan. Therefore, any legal document with the intention to bind the University of Michigan to an obligation should not be signed by U-M research faculty or staff.
Yes, there are many ways to work with industry that yield benefits for both parties. These fee-for-service agreements can generate preliminary data which may support future sponsored research opportunities.
No, a conflict of Interest (COI) does not mean a University of Michigan investigator cannot work with industry. Any potential conflicts should be reviewed and managed by the Medical School’s Regulatory Affairs Office ([email protected]).
Yes, consulting is allowed. Typically, companies compensate investigators for their professional expertise to guide strategy or interpretation of results. University of Michigan has policies that govern consulting activities. Please watch our video to learn more or contact the FFMI BD team to facilitate.
Yes, the terms of these deals determine what type of agreement and potential committee reviews would be required. Please watch our video or visit the Data & Biospecimen Sharing website.
The BD team serves as liaisons between our external partners and University of Michigan Medical School faculty, helping navigate the many world-renowned departments and programs available at Michigan Medicine.
Work With Us

Michigan Medicine is at the forefront of partnering with sponsoring organizations on clinical trials, advancing healthcare in Michigan and around the world. View the video below to learn more about the University of Michigan’s clinical trial enterprise.

Research Areas: Immunology, GI & Hepatology, Pulmonary, Digital Health, Human Genetics, Cardiovascular

Research Areas: Clinical Oncology, Cell-based Therapy, Solid Tumors, Liquid Tumors, Biomarkers, RNA Therapeutics
Research Areas: Basic Oncology, Developmental Therapeutics, Dermatology, Psychedelics, Ophthalmology, Data & Biospecimans

Research Areas: Diabetes, Neurology, Obstetrics, Gynecology, Sleep Medicine, Basic Oncology

"I'm extremely grateful for the opportunity to work closely with the FFMI Business Development group. They listened carefully to my team and gained a full understanding of our research priorities and interests. Using that information, they leveraged industry connections to create brand new research partnerships that have accelerated our research and have been essential to the growth of our Initiative. Mike and Shelby are now integral members of our team and continue to advance our research in new and exciting ways!”

Building 520, 3rd Floor
2800 Plymouth Road
Ann Arbor, MI 48109-2800