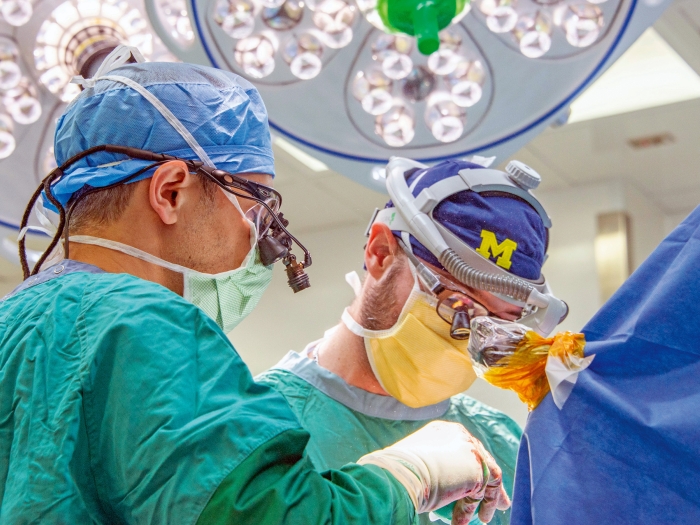
As one of the most powerful academic medical research engines in the country, the University of Michigan Medical School empowers our scientists and clinicians to work together to transform the biggest challenges in biomedicine into breakthroughs in patient care.


Groundbreaking discoveries that happen daily at the University of Michigan Medical School are made possible by our unique system of collaboration and innovation. We bring together expert researchers, clinicians and clinician-scientists across disciplines and provide them the tools, training and funding they need to make connections that are crucial to medical breakthroughs.
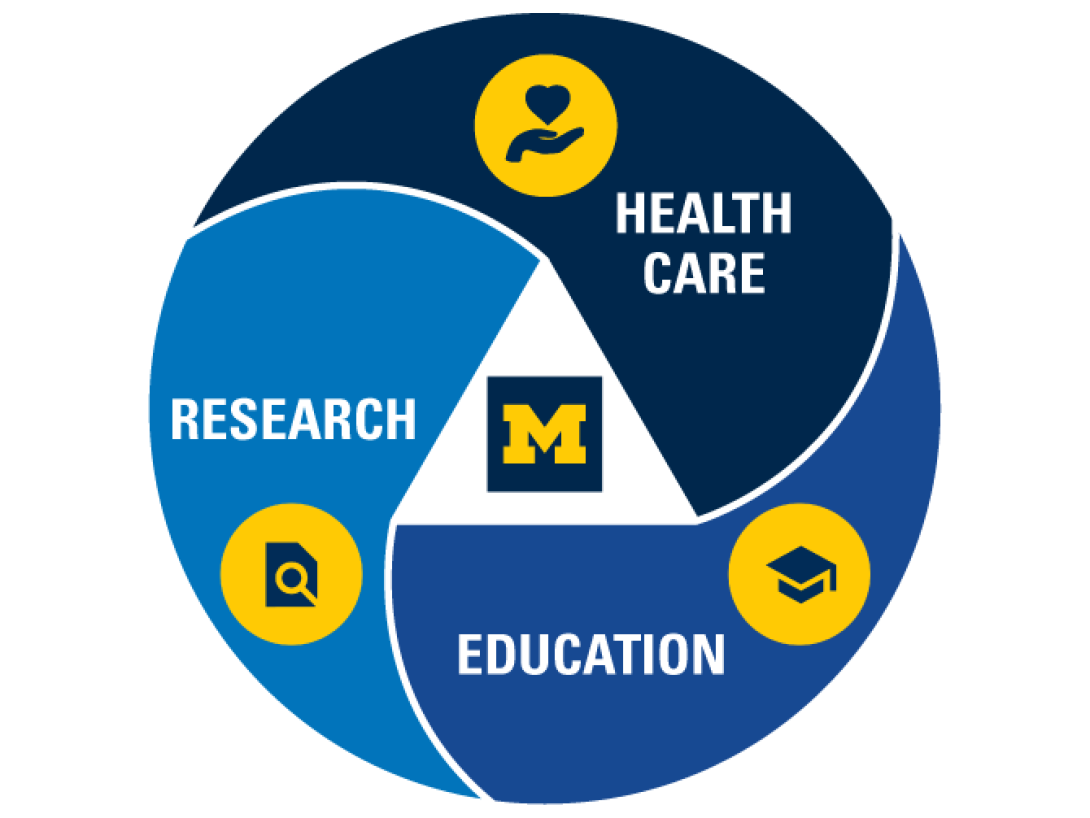
Patient needs are constantly evolving, impacting the future of health care Now more than ever, it's vital to empower diverse approaches to science and medicine. Through the U-M Medical School's interconnected research areas, we bring together experts from an array of fields to collaborate, innovate and make discoveries that transform patient lives.

Since its founding in 1850, the University of Michigan Medical School has forged a strong leadership role in American academic medicine.