
Getting Started
Follow these steps for a seamless and streamlined onboarding.
Use the guidance below to help you navigate the early steps of working with the CBR.
- Review our Regulatory and Governance policies
- Review CBR Service Rates
- Submit a Request for Prospective Services
- Complete and submit this form
- Determine your Oversight Committee members and create a charter
- As defined in the Research Biorespository Policy, all biorepositories are required to establish an Oversight Committee for their collection.
- The CBR has a template Oversight Committee Charter for use if desired. Contact Savanna Sneeringer ([email protected]) for a copy of the template.
- CBR requires the list of the Oversight Committee members for documentation.
- Update your IRB to use the CBR Consent
- Biorepository consent template
- Biorepository information pamphlet. This is a required part of the consent.
- If participation in the biorepository is optional for your subjects, you will need two consents - one for the main study and one for the biorepository (consent above).
- Full regulatory guidance is available here.
Determining and documenting your sample collection, processing, and storage plan (workflow, Excel grid, sample processing protocol, etc.) will help improve the onboarding process. We will work with you during onboarding to refine the details, but a few items to begin considering are listed below:
Collection
- What is the sample collection visit schedule?
- What samples will be collected at each visit?
- What collection tubes will be used?
- Who will be doing the collection (e.g., study team, CTSO, clinical staff)?
- If sample collection and visit schedule are different per cohort, please define.
Processing
- Will any processing be done? If so, what processing (e.g., blood fractionation)?
- Who will be executing the processing (e.g., study team, MCRU, another lab)?
- Any special considerations on processing (i.e., whole blood needs to be processed within a certain period of time to preserve the quality of the derivatives)?
- Aliquots/Derivatives: What child sample types will be created from each parent? How many? What volume?
Storage
- What are the long-term storage conditions for each sample type?
- What samples will be sent to the CBR? Will any be stored in your lab or sent immediately to another location?
Storage conditions should primarily be based on the needs of the research. However, CBR has some storage recommendations and vial requirements.
Required Specs for Cryovials
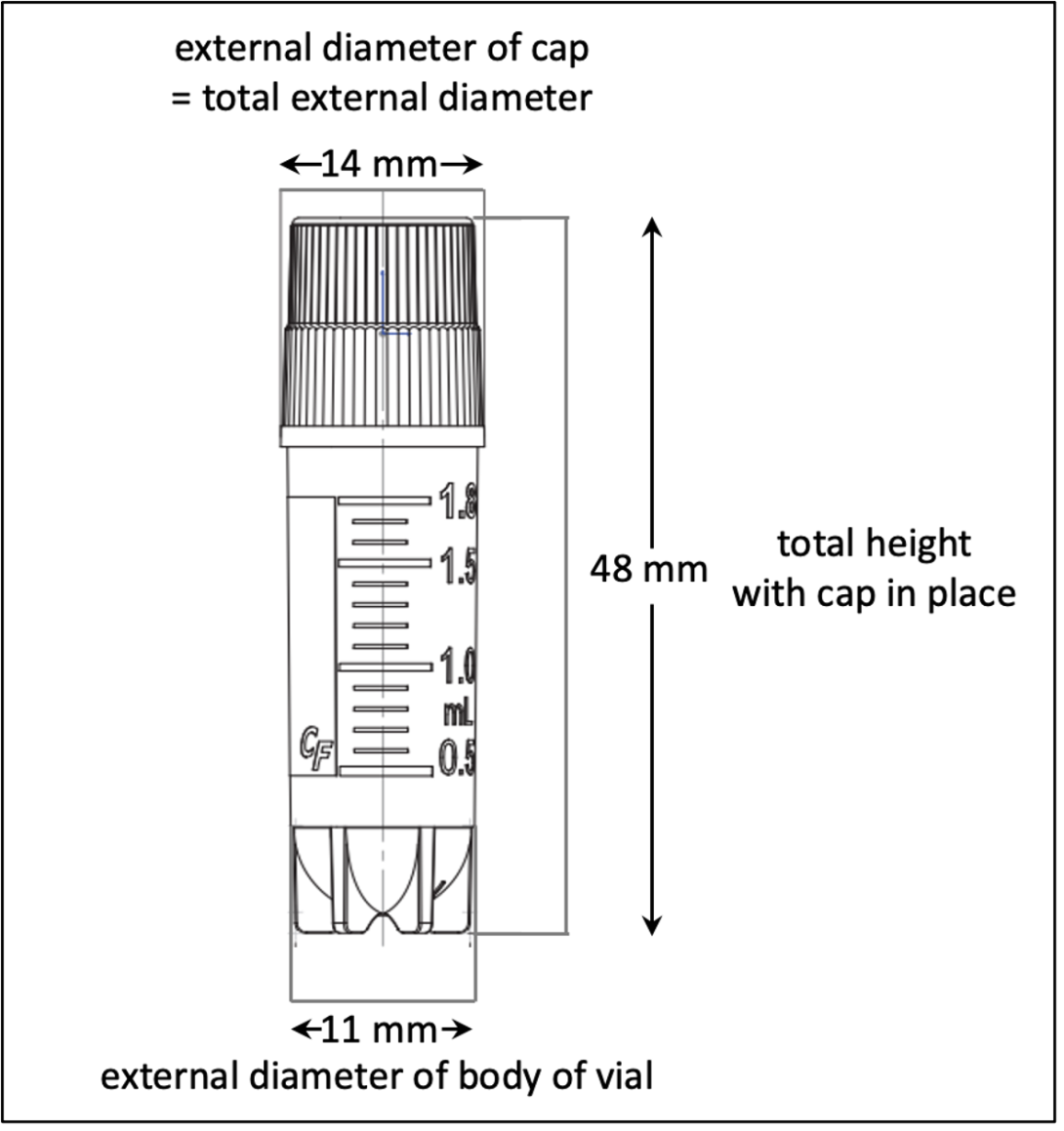
- Must be rated for the appropriate storage temperature. CBR's coldest freezers are chilled by Liquid Nitrogen (LN) Vapor, -196°C. Cryovials are not submerged in LN at the CBR.
- Externally threaded, O-ring caps are REQUIRED. Flip-top tubes will not be accepted.
- Free-standing cryovials are preferred.
- Size specifications for 2.0ml cryovials holding biospecimens destined for automated aliquoting or automated DNA extraction:
- the external diameter of the body of the vial may not exceed 11mm
- the total external diameter of the cryovial with the cap in place may not exceed 14mm
- the total height of the cryovial with the cap in place may not exceed 48mm
- Size specifications for cryovials holding biospecimens destined for storage-only and/or whole-vial distribution:
- 0.5ml cryovials should have the same external dimensions as 2.0ml cryovials
- 2.0ml cryovials with caps in place may not exceed a total external diameter of 14mm AND may not exceed a total height of 48mm
- 4.5-5ml cryovials with caps in place may not exceed a total external diameter of 14mm AND may not exceed a total height of 91mm
Recommended Storage Conditions
- In general, the cooler the better. For example:
- DNA: -80°C
- Plasma/Serum: LN2 Vapor, unless scientific reason to store otherwise
Other
- Barcoded label with unique identifiers for each sample
- Will you be consenting pediatric patients to the CBR?
- If so, what is your plan to reapproach/re-consent the pediatric participants when they turn 18?
- Will you have the option for a Legally Authorized Representative to consent on behalf of a participant?
- If so, do you have a plan to directly consent the participant at a later date?
- Do you intend to establish a partnership with Precision Health (Michigan Genomics Initiative)?
- Have you received approval from Precision Health?
- Have you updated your consent to include MGI-specific language?
- For questions, contact Janet Houghtby ([email protected]).
- If you have samples collected prior to onboarding with the Central Biorepository, a retrospective collection review and approval process will be required. Please complete the Retrospective for Retrospective Collection Transfer form in addition to the Request for Prospective Services form.
2800 Plymouth Road
Ann Arbor, MI 48109
Hours of Operation: 7:30 am - 4:30 pm, Monday - Friday
Want to provide feedback? Click here to access a brief survey about your CBR service.

We're Accredited – The Central Biorepository is accredited by the College of American Pathologists (CAP) Biorepository Accreditation Program.